Archeomalacology: What We Can Learn from Snails

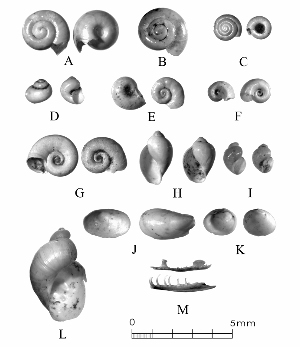
This is science, but it’s about as low-tech as you can get. Preliminary sorting of microsnails can be done without magnification, but final identification requires a microscope, usually at about 10-30X. Specimens are manipulated with an artist’s paintbrush, or sometimes with a pair of Italian steel forceps. Photo by Ken Brown. |
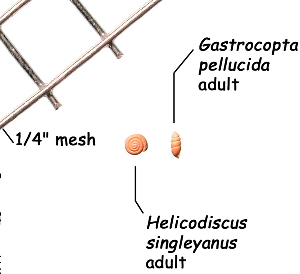
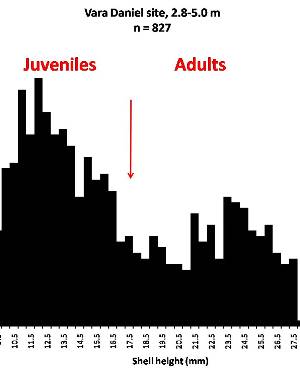
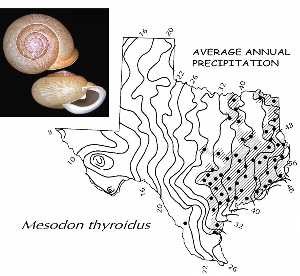
Sometimes even the smallest of creatures can provide big insights. Ken Brown, an archeologist and TARL Research Affiliate, has been on the trail of snails for decades. From these seemingly inconsequential mollusks, a wealth of data about past environments at archeological sites can be gleaned. Simply put, different species of snails thrive in different environmental conditions. By determining which species were present at a given time, he can reconstruct what the climatic conditions were like over time. His observation of snails (species, habitat requirements, species densities and distributions, etc) allow him to contribute invaluable insight into site formation and transformation over time, evidence of bioturbation, possible signs of cultural utilization, and especially paleo-environmental reconstruction. The processes involved in snail analysis are painstaking and often tedious, involving screening sediment samples through a series of increasingly fine-grained mesh, picking out the shells, sorting by size, and finally identifying them. Good eyesight is a must for this job: some snails in his samples are less than a millimeter across, and specimen storage is in gel caps, not plastic bags. For archeologists, however, this sort of information can be invaluable—and often the only available indicator of past environments. By Ken Brown Archeomalacology is the study of mollusks in archeological contexts. Strictly speaking, this might include marine bivalves, marine snails, freshwater mussels, and various kinds of inland snails. In practice, though, I work only with snails (terrestrial, amphibious, and aquatic) from continental settings, along with some other kinds of very small invertebrate organisms that are sometimes recovered in snail sampling (peaclams, fingernail clams, and freshwater limpets). There are two reasons why archeologists might want to commission studies of snails from archeological sites: 1) Snails are useful paleoenvironmental indicators. 2) In Central and South Texas, snails of the genus Rabdotus were a conspicuous food item beginning in the Early Archaic and perhaps peaking in exploitation in the Late Archaic. Furthermore, snails can be used as a source of organic material for radiocarbon assay or epimerization studies, and have also been used for carbon and oxygen isotope studies. Although to most archeologists, “snail” and “Rabdotus” are synonymous, in reality there are many native Texas land and amphibious species and perhaps as many as 41 aquatic species (although DNA studies are collapsing this number). Kathryn Perez estimates that there are as many as 185 contemporary and extirpated terrestrial species and subspecies, although I am skeptical that all these of these species reports are valid (many reports probably date from decades ago, when taxonomic splitting was rampant, and the real number of Texas natives is probably significantly lower). There are also a few species that have been extirpated since the Pleistocene, and around a dozen or so invasive Eurasian land or aquatic species. The native terrestrial species differ widely in habitat preference and body size, from the tiny Carychium mexicanum (adult shell height, 1.7-2.0 mm) to Rabdotus alternatus (adult shell height, up to 4.3 cm). In archeological sites where habitats were favorable and proper sampling is done, generally about two to three dozen taxa can be expected. In Texas, the Lubbock Lake site holds the record for diversity, with just under four dozen taxa. Snail shell usually preserves well anywhere in Texas except in sandy, acidic, calcium-deficient soils in east Texas (or clay-rich vertisols). Bone is about 30% organic material (collagen), and when bone decays, bacteria attack the organic component, opening up the matrix to further degradation by soil acids. Snail shell, on the other hand, has only about 1-5% organic matter or less, and is essentially immune to bacterial attack. I have seen a number of Texas archeological sites where the bone was completely decayed away, or reduced to corroded, unidentifiable fragments, but the snail shell was well preserved. A good example is furnished by the Paleoindian deposits at the Pavo Real site, where almost no bone whatsoever was recovered, but where snail shell was well preserved, and the assemblage was small only because the excavators made no effort at systematic collection. Most Texas archeologists have no interest in snails whatsoever. The standard research protocol for snails is to throw them away. I have seen many reports from snail-rich parts of Texas in which the word “snail” never appears anywhere, even in stratigraphic descriptions. In CRM studies, snails are one more type of analysis that must be paid for, and another collection category that must be curated. Better to pretend they don’t exist— that’s the prevailing paradigm. Although basic scientific terminology for mammals became solidified by the beginning of the 20th century, the situation for snails is very different. The taxonomy is constantly changing, and archeologists should be aware of that. In the war between lumpers and splitters, the splitters carried the day in the 1930s-1960s, and as a result, a great many species and subspecies were created that are now known to be invalid (perhaps this is why so many species have been reported for Texas). In the last couple of decades, biologists have started to apply DNA analysis to snails, and as a result, many taxa have been collapsed into simpler groupings, often with revised names. As a result, one needs to understand the taxonomic history to correlate new species names with the old ones, because many of the snails reported in the Texas archeomalacological literature are listed under old, outdated names. The example probably most familiar to archeologists is the revision in 1974 of the genus Bulimulus to the genus Rabdotus, but there are many other examples to be found, and every year the species names change. Snails are highly moisture-dependant, which makes them good paleo-moisture proxies in archeological sites. Unlike cursorial mammals, they can’t travel to a water source, and when they do travel, it’s on a mucus trail that results in major water loss. Everyone has probably seen the slime trails left by slugs on rainy nights. Slugs are snails that have lost their external shell through evolutionary processes. Active slugs can lose 30-40% of their body weight within two hours. Snails regulate water loss by adjusting behavior in both time and space. They are nocturnal, they gravitate towards areas with high relative humidity or moist substrates, and they are inactive during the day. In dry periods, snails can estivate, practicing metabolic arrest and tolerating remarkably high levels of toxins in their kidneys, withdrawing into the shell and sealing it with an epiphragm. Most snails are not particularly temperature-sensitive, but they are useful indicators of past moisture conditions and microhabitats. This can readily be seen on a macro scale by plotting their geographic ranges on isopleth maps of annual rainfall. When archeologists identify animal bones to try to diagnose past environments, they are essentially “sampling” an area that may have a radius anywhere from meters to kilometers in size. With buried pollen, the sampling radius may be as much as tens or hundreds of kilometers, depending on whether the plants were wind-pollinated or insect-pollinated. With snails, the sampling radius is immediate to the site area, unless they have been transported downstream long distances and deposited in alluvium. The smallest snail species have a home range that may only be a few tens of centimeters in radius, so snails can often provide information on past habitats that are much more localized in geographic scope. This can be an advantage if the project archeologist is more interested in the site paleoenvironment than the regional paleoenvironment. Sampling of snails for paleoenvironmental studies has much in common with the procedures used for charcoal and plant macrofossils, although there are some differences. Because archeologists are usually concerned with environmental change over time, columns of samples at 5-cm or 10-cm intervals are collected from standing profile walls (preferably one already recorded by the project geomorphologist). The sampling intervals are tied into the site grid, usually shot in with a total data station, and often the levels are the same as those used in the excavation units. While in theory we could just as easily sample horizontally on a given surface, it’s usually change over time we’re looking for. My target sample size is usually 15-20 liters of sediment. These are large samples, much larger than those used in pollen analysis. The goal is to obtain specimen counts large enough to allow quantitative treatment of data. Sediment samples are dried, weighed (in kilograms), measured volumetrically (in liters), then soaked overnight in water (with sodium carbonate if clay-rich). If snails float to the surface, they are scooped up with a strainer and dried separately (as “floaters”). The sediment is wet-sieved through nested 2 mm, 1 mm, and 0.5 mm brass geologic sieves, without pushing the sediment through the sieve. These are custom-made, extra-large sieves, 18 inches in diameter to allow processing of the oversize samples. Residue retained on the sieves is dried in the lab, then manually picked with forceps or a paintbrush. Microsnails are identified under low magnification (usually only about 10X, but up to 40X). I also save shell fragments and weigh them, because these can indicate changes over time in taphonomic conditions, and because some taxa can be identified even from fragments. Many archeologists seem to think that only complete shells can be identified and need to be saved, but this is not true. It depends on the species. The specimen counts and weights are then entered in spreadsheets. Photodocumentation is done, generally with a microscope-mounted videocam for small specimens. Microsnails are stored in gelcaps placed in plastic vials (not bags!). I also save any other kinds of material that may be of interest to other specialists working on a project. Vertebrate bone (including rodent teeth, fish bone, and snake vertebrae), fish scales, charcoal fragments, charred seeds, uncharred hackberry seeds, flakes of mussel shell, microdebitage from stone toolmaking, ostracod valves, and charophyte oogonia (the calcareous fruiting bodies from green algae) can emerge from the snail samples. At the Genevieve Lykes Duncan site, even the sediment residue itself was saved for heavy mineral separation studies. As a very rough estimate, perhaps 30% of the native Texas land species are large-bodied (adult shell diameter over about 10 mm), perhaps another 24% or so are medium bodied (about 5-10 mm diameter), and the remaining 46% are microsnails (these percentages are somewhat problematical). Aquatic snails also have a wide range of body sizes, although the size partitioning is unknown. All of these are potentially informative about past environments (especially the microsnails), so a sampling technique that recovers the full spectrum of body sizes is important. A reasonable guess would be that about 60% of the adult terrestrial species in Texas are too small to be retained on standard quarter-inch field screens. That means that in any particular site, not a single example of about 60% of the species that are actually present might show up unless fine sieving is done. The situation is even worse when we consider juveniles. All juvenile microsnails, perhaps half or more of the medium-bodied species, and even many of the large-bodied species will fall through standard quarter-inch mesh. Because column samples, even 20-liter ones, tend not to capture very many large-bodied snails, I like to combine that information with a sample of large and medium-bodied snails from field screens. I will usually ask the field crew to save all snails and shell fragments from one or two excavation units that are closest to the column sample, and if they complain, I can always bribe them with the promise of cold beer. Analysis involves identifying each specimen to species, genus, or family if possible; tabulating adults and juveniles, complete and partial specimens separately; and measuring selected species (to the nearest tenth of a millimeter). While it’s not always possible to identify each specimen to the species level, it may not matter. Often, the members of a genus or even of a family share habitat preferences sufficiently that it’s adequate just to pin down the ID to the genus or family level. The real goal of identification is to see what habitat preferences are expressed in the assemblage at any one level, and how these might change over time, because the habitat preferences tell us what the environment was like. If there’s consensus among the different snail species, we know there was a uniform environment. If there are disparities, we know we either have a mosaic or patchy environment, or else transport mechanisms (like flooding) have introduced snails from contrasting habitats. When identification is finished, we can look for changes in faunal composition over time, plot stratigraphic changes in adult-juvenile ratios, use the metric data to look for secular changes in body size, and plot changes in species richness and specimen abundance, as well as ratios of complete and fragmentary specimens. Experience with Texas sediments has shown that depositional history is a major driver of changes in snail faunas. In alluvial deposits, it seems to be common for snail species richness and abundance to increase over time and with diminishing depth, because the deepest deposits show evidence of higher depositional rates. Likewise, the percentage of aquatic species may decline over time, not so much because the environment is becoming drier, but because the terrace tread is building up and as time goes on, it is less likely that flooding will deposit aquatic snails on the surface. Consequently, the aquatic/terrestrial ratio declines over time. Archeomalacology is both science and art. The art lies in recognizing these intrinsic geologic drivers of change and parsing out the less parochial changes that represent the regional environment, or site-specific microhabitats. If sample columns are deep enough to penetrate Pleistocene sediments, they may document significant extirpation events or range changes at the end of the last ice age. At the Vara Daniel site (Travis County), the terrestrials Cochlicopa lubricella, Pupilla muscorum, Vallonia sp., and Pomatiopsis lapidaria, and at Berger Bluff (Goliad County) the aquatic Valvata tricarinata appeared to be potential Pleistocene marker species. In the San Antonio area, Anguispira strongylodes appears at Pavo Real and at the San Antonio River Mammoth site, well beyond its current range. Since the end of the Pleistocene in Texas, the history of snail faunas has been one of reduced biodiversity, range retraction to the eastward (or to higher altitude in mountainous west Texas, or to the more extreme case of retraction: statewide extirpation), expansion of neotropical taxa, and in historic times, the invasion of Eurasian species. Among the aquatic snails, pulmonate species (lung-breathing snails capable of surviving in stagnant, poorly oxygenated waters) probably survived Holocene drying better than prosobranch (gill-breathing species which prefer clear-running, well-oxygenated water) snails. In Texas, paleoenvironmental studies of snails have been done at early sites like Lubbock Lake, Lake Theo, Rex Rodgers, Wilson-Leonard, Vara Daniel, the Aubrey site, Richard Beene site, the bench deposits at Berger Bluff, La Paloma Mammoth site; and at later Holocene sites like the Anthon site, Fish Creek Slough, Guadalupe Bay, Kenyon Rockshelter, Smith Creek Bridge site, and the Hog Creek sites. These low-tech studies are difficult and labor-intensive, but they can be done most places in the state, especially where other paleoenvironmental proxies are scarce or lacking, and require little in the way of expensive equipment. The wide applicability of these studies would seem to warrant better attention from Texas archeologists in the future. Project archeologists contemplating such studies should contact the archeomalacologist well in advance and arrange for a site visit, preferably after good profiles have been developed, and as the project geomorphologist finishes fieldwork. Sample collection by the malacologist is preferable to samples collected by the field crew, except for selected field screen samples. Close consultation with the geomorphologist is essential. Viewers who want to know more about archeomalacology can look for Snails: Archaeology and Landscape Change (Paul Davies, 2008, Oxbow Books) or peruse online back issues of the ArchaeoMalacology Newsletter.
|
|